Europlaz has been a trusted provider of design, engineering, and manufacturing expertise for the medical device industry for over 50 years. During this time, we have developed a comprehensive service that supports the entire life cycle of a medical device, from concept design to manufacturing and quality assurance. With our flexibility, resourcefulness, and experience working with clients of all sizes, we can meet the unique needs of your business.
The Europlaz 5-step medical device design process
Designing and developing medical devices is a complex process that requires planning, an understanding of patients’ needs, cutting-edge facilities, and experience. Our focus lies in producing the highest quality plastic injection moulded parts, sub-assemblies, and finished medical devices.
But what does this process look like? And how can you plan for your future success? Allow us to take you through the Europlaz planning process to see how we provide insight, expertise, and support throughout your medical device design process.
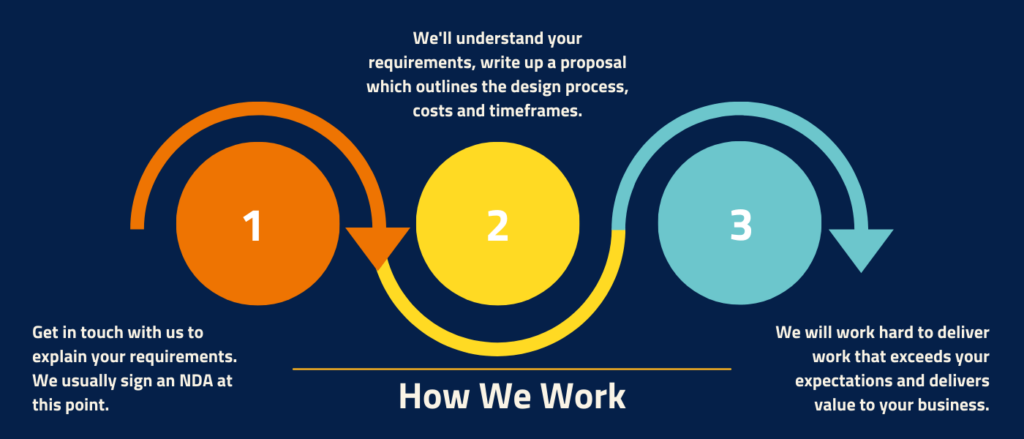
Step 1: project planning

In this phase, we take the time to understand your project and requirements. We work closely with you to determine the scope of work and create a detailed project plan. This includes defining the project objectives, establishing timelines, and identifying key milestones.
Step 2: concept definition & feasibility studies

Once the project plan is in place, we move on to generating early design concepts and prototypes. This step allows us to assess the feasibility of your medical device idea. We leverage our expertise and equipment to develop innovative designs that are both technologically and commercially viable.
Step 3: prototyping & validation

Prototyping is a crucial phase of the medical device design process where we transform design concepts into physical prototypes. These prototypes undergo rigorous testing to ensure that they meet the specified requirements, user needs, and intended use.
Step 4: production

Once we have a working prototype, we transition from prototyping to production. We generate work instructions, validate assembly processes, and complete technical documentation required for regulatory submissions. Our experienced team ensures that all regulatory standards and requirements are met to facilitate a smooth market entry for your medical device.
Step 5: manufacturing at scale

Once the pilot production is completed, we move into full-scale manufacturing. Europlaz operates within an ISO 13485 and FDA-registered environment, ensuring the highest quality standards are maintained throughout the manufacturing process.
We have extensive experience in medical device design and manufacturing processes and work with specialised medical plastic injection moulding machines to assemble millimetre-perfect products.
Decades of experience in the medical device design process
As shown by the above, we have extensive experience in medical product development and access to cutting-edge technologies and equipment. Whether working on single-use medical test kits or bespoke medical application equipment our team can help transform your medical device idea into an innovative design that is both technologically advanced and commercially viable.
To support this, we adhere to regulatory requirements to ensure every product that leaves our doors is robust and trustworthy. We prioritise quality by adhering to a rigorous Quality Management System (QMS) based on ISO 13485:2016, the European Medical Device Directive 93/42/EEC, and FDA 21 CFR part 820 Quality System Regulations for medical devices. Our teams also adhere to internal quality objectives to uphold the highest quality standards throughout the manufacturing process.
Work with Europlaz today
As you can see, we specialise in producing the highest-quality medical plastic injection moulded parts, sub-assemblies, and finished medical devices through a dedicated process from design to rollout. As a result, we are proud of our reputation within the medical sector for delivering exceptional quality and durability.
If you are interested in finding out more about the process above or want to get started on your next medical device project contact us today. Our team is ready and waiting to help you plan, design, and create medical devices that help patients every day.





