How we work
Helping you to achieve cost effective, high quality manufacture.
What we do
Over the last 50 years, Europlaz has provided design, engineering and manufacturing expertise for the medical device industry.
We work across the entire life cycle of a product: from concept design, to manufacturing, to after-market services.
We are flexible, and resourceful, and can take on projects of any size. Our clients range from small start-ups to international blue chip medical device suppliers.


Different ways we can work with you
Whether you are looking for a complete turnkey solution, or are just supplementing your internal capabilities, Europlaz has the background and flexibility to meet your needs. We specialise in producing the highest quality plastic injection moulded parts, sub-assemblies and finished medical devices.
The medical device design and development stage involves complex processes that Europlaz aims to make as simple as possible for you. We can manage all aspects from the specification of the tooling, tool commissioning, first samples, validation, production of batches for clinical trials, in house tool modifications – all the way through to the supply of large batches ready for clinical use.
Manufacturing Excellence for Healthcare Innovation.
Helping OEMs and Inventors to scale their businesses by partnering with a flexible medical device contract manufacturer.
Want to find out more?
Areas of expertise
1. MEDICAL DEVICE DEVELOPMENT
We are experienced in product development and have access to world-class technologies and equipment. We can help you turn your medical device idea into an innovative design, and then into a technologically and commercially viable product.
2. MEDICAL DEVICE REGULATION
The medical device industry is highly regulated, and the regulations are constantly changing. We have experience in the UK, European, American and Asian markets, and can provide regulatory support to help you smoothly enter your medical device into the relevant market.
3. QUALITY ASSURANCE
We uphold our quality standards through a rigorous QMS based on the principles of ISO13485:2016, the European Medical Device Directive 93/42/EEC, the FDA 21 CFR part 820 Quality System Regulations for medical devices, and the establishment and delivery of related quality objectives.


Areas of expertise
4. CLEANROOM MEDICAL DEVICE MANUFACTURING
We specialise in producing the highest-quality plastic injection moulding parts, sub-assemblies and finished medical devices. We have expert knowledge of materials and manufacturing processes, we well as long-standing partnerships with machining and tooling experts
5. INJECTION MOULDING & ASSEMBLY
Europlaz is highly experienced in getting the tooling and injection processes for medical devices just right – building a reputation within the medical sector for quality and durability.
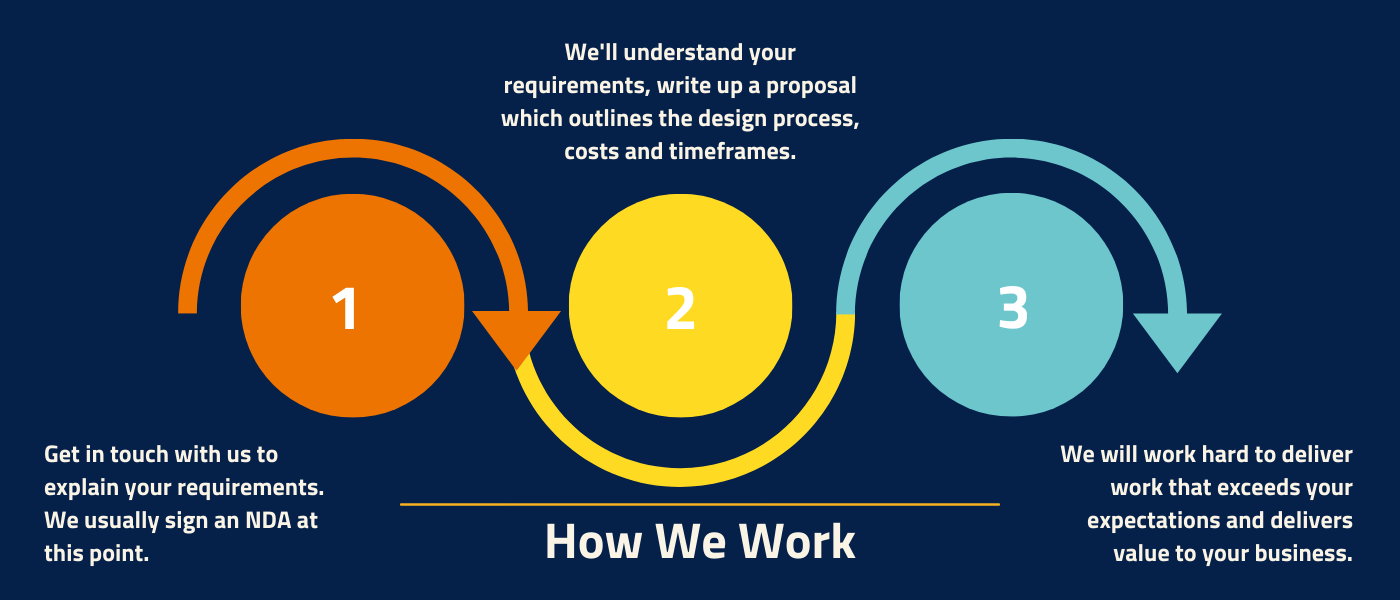
How we work
When working with Europlaz our process follows 3 simple steps to help you achieve cost-effective, high-quality manufacture.
Partnering with Europlaz
When partnering with Europlaz we aim to make the process as smooth and transparent as possible, as detailed below.
To discuss the benefits of working with Europlaz please contact us today.
Step 1: Project planning
Taking the time to understand your idea, determine the scope of work and create a project plan.


Step 2: Concept definition & feasibility
Generating early design concepts and prototypes to establish feasibility.
Step 3: Prototyping & validation
Conducting testing on prototypes to ensure the device meets the specification, user needs and intended use.


Step 4: Pilot production
Generating work instructions, validating assembly processes, completing technical documentation & regulatory submissions.
Step 5: Volume manufacturing
Full scale manufacturing within an ISO 13485 and FDA registered environment.

About Europlaz
Since 1970 we have built a reputation for innovation, quality, and cost-effectiveness.
We use our experience and state-of-the-art technology to streamline all stages of the medical device manufacturing process for you – moving your product quickly and safely through development, and speeding up regulatory approval of medical devices.
Our UK-based ISO 13485 certified and FDA-compliant facility means that we have complete control of the process, from under one roof. We are flexible, and resourceful, and can take on projects of any size. Our clients range from small start-ups to international blue chip medical device suppliers.
For further information you can watch our Corporate Video to the left-hand side, view our Corporate Brochure or Contact Us.
Book a Meeting
To learn more about Europlaz’s services and the different medical devices we have helped develop, book a telephone meeting with one of the team.
To do this simply use the Schedule Meeting button below.
Alternatively you can contact us by telephone or email.
